2. 5 Perfect Isotonic Solution Designs For Your Experiments Today
Creating the right isotonic solution is crucial for various scientific experiments, especially when working with cells and tissues. An isotonic solution maintains the balance of solutes and water, ensuring optimal conditions for cellular processes. In this blog post, we will explore five carefully crafted isotonic solution designs that will enhance the success of your experiments.
1. The Classic Physiological Saline Solution

The physiological saline solution is a staple in many laboratories. It closely mimics the ionic composition of human blood plasma, making it an excellent choice for a wide range of biological experiments. Here's a simple recipe to prepare this isotonic solution:
- Ingredients:
- 0.9% Sodium Chloride (NaCl)
- Distilled Water
- Preparation:
- Dissolve 9 grams of NaCl in 1 liter of distilled water.
- Stir until the salt is completely dissolved.
- Adjust the pH to 7.4 using a pH meter or pH indicator paper.
- Filter the solution to remove any impurities.
This solution is isotonic to human cells and can be used for cell culture, tissue preservation, and various physiological studies.
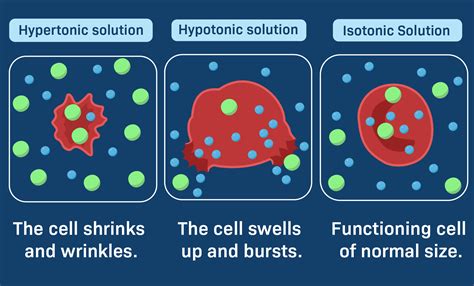
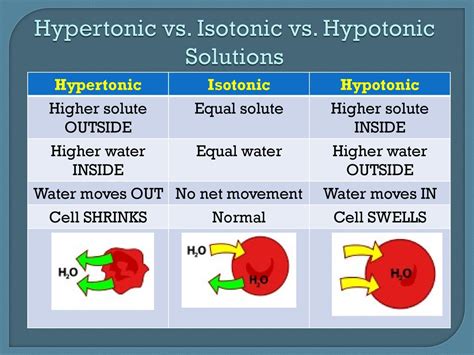
2. Hypotonic Solutions for Cell Swelling

In some experiments, you may want to induce cell swelling to study specific cellular responses. Hypotonic solutions have a lower solute concentration than the cell's internal environment, causing water to enter the cell. Here's a recipe for a hypotonic solution:
- Ingredients:
- 0.45% Sodium Chloride (NaCl)
- Distilled Water
- Preparation:
- Dissolve 4.5 grams of NaCl in 1 liter of distilled water.
- Mix well and adjust the pH to 7.4.
- Filter the solution to ensure purity.
Using this hypotonic solution, you can observe the effects of cell swelling on cellular processes and membrane dynamics.
3. Hypertonic Solutions for Cell Shrinkage

Conversely, hypertonic solutions have a higher solute concentration, leading to cell shrinkage as water moves out of the cell. This type of solution is useful for studying cell volume regulation and osmoregulation. Here's a recipe for a hypertonic solution:
- Ingredients:
- 1.8% Sodium Chloride (NaCl)
- Distilled Water
- Preparation:
- Dissolve 18 grams of NaCl in 1 liter of distilled water.
- Stir until the salt is fully dissolved.
- Adjust the pH to match the experimental requirements.
- Filter the solution to remove any impurities.
Hypertonic solutions are valuable tools for investigating cell adaptation and survival mechanisms under stressful conditions.
4. Customized Isotonic Solutions for Specific Cell Types
Certain cell types, such as red blood cells or plant cells, have unique ionic requirements. In such cases, you can customize isotonic solutions to match the specific needs of these cells. Here's an example for a red blood cell isotonic solution:
- Ingredients:
- 0.9% Sodium Chloride (NaCl)
- 0.2% Potassium Chloride (KCl)
- 0.025% Magnesium Sulfate (MgSO4)
- 0.02% Calcium Chloride (CaCl2)
- Distilled Water
- Preparation:
- Dissolve the salts in the specified amounts in 1 liter of distilled water.
- Stir thoroughly and adjust the pH to 7.4.
- Filter the solution to ensure it is free from contaminants.
This customized isotonic solution is ideal for maintaining the integrity of red blood cells during experiments.
5. Buffer-Based Isotonic Solutions for pH Control

When precise pH control is essential, buffer-based isotonic solutions are the way to go. These solutions maintain a stable pH while providing an isotonic environment for cells. Here's a recipe for a phosphate-buffered saline (PBS) solution:
- Ingredients:
- 0.01M Sodium Phosphate Monobasic (NaH2PO4)
- 0.01M Sodium Phosphate Dibasic (Na2HPO4)
- 0.137M Sodium Chloride (NaCl)
- Distilled Water
- Preparation:
- Dissolve the sodium phosphates and NaCl in the specified amounts in 1 liter of distilled water.
- Adjust the pH to 7.4 using a pH meter.
- Filter the solution to remove any undissolved particles.
PBS is widely used in cell culture, immunology, and molecular biology experiments due to its pH stability and isotonic properties.
Choosing the Right Isotonic Solution

When selecting an isotonic solution for your experiments, consider the following factors:
- Cell Type: Different cell types have unique ionic and osmotic requirements. Ensure the solution matches the needs of your specific cells.
- Experimental Goals: Determine whether you aim to maintain cell integrity, induce swelling or shrinkage, or control pH. Choose a solution that aligns with your research objectives.
- pH Stability: Some experiments require a stable pH environment. Buffer-based solutions like PBS are ideal for maintaining pH balance.
- Purity: Always use high-quality, filtered solutions to avoid introducing contaminants that could affect your experimental outcomes.
Tips for Preparing Isotonic Solutions
Here are some additional tips to ensure the successful preparation of isotonic solutions:
- Accuracy: Use precise measuring equipment to ensure the correct concentration of solutes.
- Stirring: Stir the solution thoroughly to ensure complete dissolution of salts.
- pH Adjustment: Always adjust the pH to the desired level using a pH meter or indicator paper.
- Filtration: Filter the solution to remove any impurities or undissolved particles.
- Storage: Store prepared solutions in a cool, dry place to maintain their stability and effectiveness.
Final Thoughts
Isotonic solutions are essential tools in the field of experimental biology, enabling researchers to create controlled environments for cellular studies. By understanding the different types of isotonic solutions and their applications, you can design experiments with greater precision and accuracy. Whether you're working with physiological saline, hypotonic, hypertonic, or customized solutions, these carefully crafted designs will contribute to the success of your scientific endeavors.
What is an isotonic solution, and why is it important in scientific experiments?

+
An isotonic solution has the same osmotic pressure as the cells or tissues it interacts with. This balance ensures that water movement across cell membranes is minimal, maintaining cellular integrity and function. Isotonic solutions are crucial for experiments involving cells, as they create a stable and controlled environment, allowing researchers to study cellular processes without the interference of osmotic stress.
Can I use tap water instead of distilled water for preparing isotonic solutions?

+
No, it is highly recommended to use distilled water for preparing isotonic solutions. Tap water may contain impurities, minerals, and microorganisms that can affect the accuracy and reliability of your experiments. Distilled water is free from these contaminants, ensuring a pure and controlled environment for your cells.
How do I know if my isotonic solution is effective for my specific cell type?

+
To ensure the effectiveness of your isotonic solution, you can perform a simple cell viability test. Incubate your cells with the solution for a specified period and assess their health and function using techniques like cell counting, viability assays, or microscopic examination. If the cells maintain their integrity and perform as expected, your solution is likely suitable for your experimental needs.